Triflic anhydride, also known as trifluoromethanesulfonic anhydride, is a highly reactive chemical reagent widely used in organic synthesis. This guide aims to provide detailed information about triflic anhydride, including its chemical properties, uses, mechanisms, handling precautions, and more.
What is Triflic Anhydride?
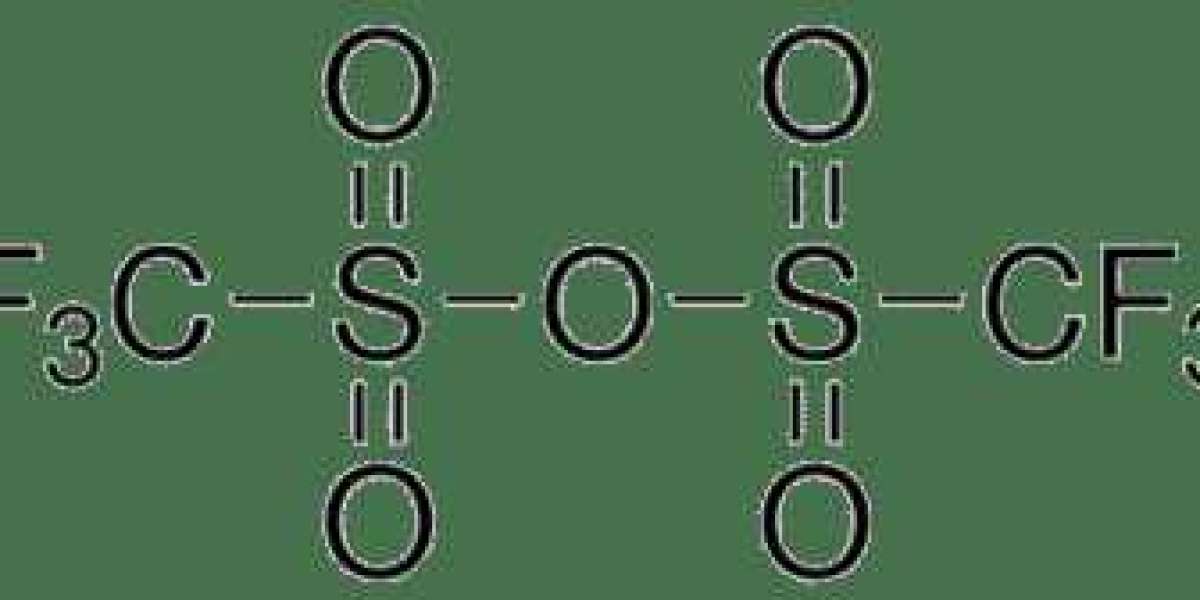
Triflic anhydride (Tf2O) is a powerful reagent utilized in various organic reactions, particularly for activating and transforming functional groups. Due to its strong electrophilic nature, it plays a crucial role in processes like acylation, sulfonation, and more.
Chemical Properties
- Chemical Formula: (CF3SO2)2O
- Case No:358-23-6
- Molecular Weight: 282.13 g/mol
- Appearance: Colorless to pale yellow liquid
- Boiling Point: 81-83°C (at 20 mmHg)
- Density: 1.675 g/cm³ at 25°C
- Solubility: Reacts with water; soluble in most organic solvents such as dichloromethane and chloroform.
Manufacturer
Triflic anhydride is produced by various chemical manufacturers who specialize in reagents for laboratory and industrial applications(Lifechempharma). These manufacturers follow strict quality control protocols to ensure high purity and consistency of the product.
Uses of Triflic Anhydride
Triflic anhydride is used in a variety of applications:
- Activation of Alcohols and Amines: It converts alcohols and amines into more reactive triflates and sulfonamides, respectively, facilitating further chemical transformations.
- Dehydration Reactions: Used to promote the formation of anhydrides and other dehydrated products from acids.
Cyclization and Rearrangement Reactions: Plays a key role in various cyclization processes and rearrangement reactions to form co