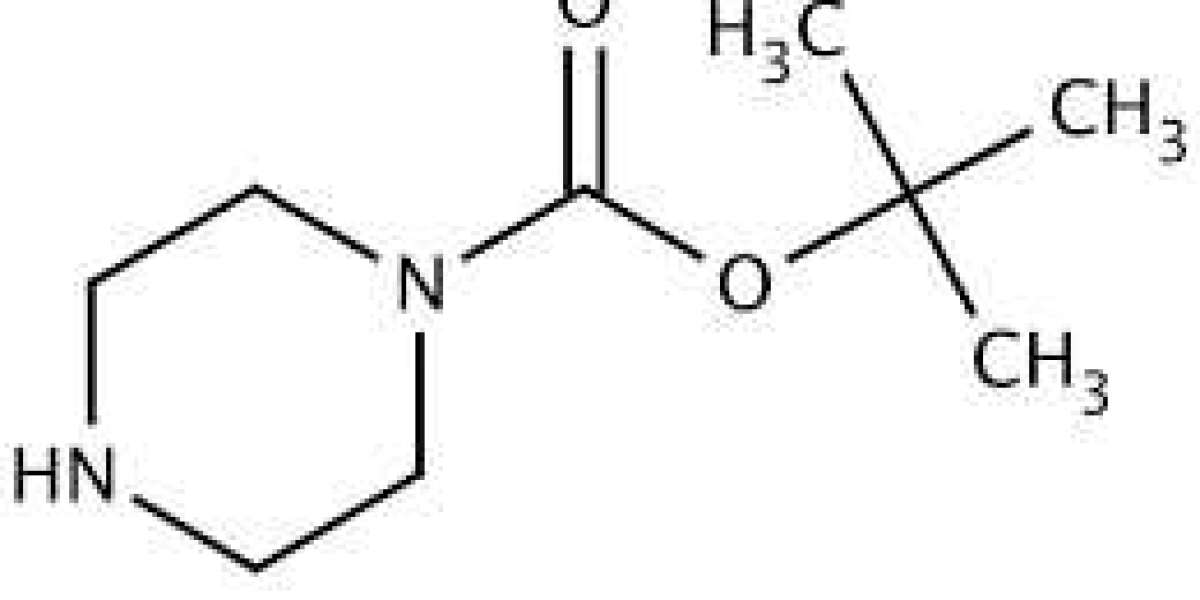
1-Boc Piperazine (N-Boc piperazine) is a widely used chemical intermediate in organic synthesis, especially in the pharmaceutical and medicinal chemistry fields. Its role as a protecting group for piperazine's nitrogen atoms is invaluable, enabling selective reactions and the development of various biologically active molecules.
Chemical Properties
- Chemical Formula: C9H18N2O2
- Molecular Weight: 186.25 g/mol
- CAS Number: 57260-71-6
- Physical State: It appears as a white to off-white crystalline powder.
- Melting Point: Around 89-91°C
- Boiling Point: Decomposes before boiling.
- Solubility: Soluble in organic solvents such as methanol, ethanol, DCM (dichloromethane), and acetone, but it has limited solubility in water.
- Purity: Commercially available with high purity levels (typically 98% or higher), essential for its use in chemical reactions.
Structural Features
1-Boc Piperazine consists of a piperazine ring, a six-membered heterocycle containing two nitrogen atoms, with one of these nitrogen atoms protected by a Boc (tert-butoxycarbonyl) group. This Boc group is bulky and prevents reactions at the protected nitrogen site, allowing chemists to control reactivity during multi-step syntheses.
Applications in Organic Synthesis
- Protecting Group for Piperazine: The Boc group is one of the most widely used protecting groups for amines due to its easy introduction and selective removal under mild acidic conditions. In piperazine, this protection is particularly useful since the molecule contains two nitrogen atoms. By protecting one nitrogen atom, the chemist can carry out selective reactions at the other nitrogen or on the carbon backbone without unwanted side reactions.
Advantages of Boc Protection:
- Stability: Boc-protected piperazine is stable under a variety of conditions, including basic and neutral environments, which makes it ideal for multi-step organic synthesis.
- Easy Deprotection: The Boc group can be removed by treatment with acids like trifluoroacetic acid (TFA) or hydrochloric acid (HCl), yielding the free piperazine for further reactions.
- Pharmaceutical and Medicinal Chemistry: 1-Boc Piperazine is a common intermediate in the synthesis of a wide variety of pharmaceuticals, including drugs targeting neurological disorders, cardiovascular conditions, and anti-infective therapies. The piperazine scaffold is often incorporated into the structure of drug molecules due to its ability to interact with biological receptors.
Example Pharmaceuticals:
- Antipsychotic Drugs: Piperazine moieties are present in several antipsychotic agents, such as aripiprazole.
- Antihistamines: Some second-generation antihistamines, like cetirizine, incorporate a piperazine ring in their molecular structure.
- Anti-parasitic Drugs: Piperazine derivatives are also used in the treatment of parasitic infections.
- Peptide Synthesis: In peptide chemistry, Boc protection is often used to protect amines in amino acids and peptide chains during solid-phase synthesis. This allows selective modifications at specific positions, a critical step in developing complex peptides or peptidomimetics.
- Cross-Coupling Reactions: The Boc-protected nitrogen in 1-Boc Piperazine prevents unwanted side reactions during cross-coupling reactions, such as Suzuki or Buchwald-Hartwig aminations. These reactions are frequently employed to form carbon-nitrogen bonds in drug synthesis, allowing for the development of nitrogen-containing heterocycles and more complex molecular architectures.
Handling and Safety Considerations
- Storage: 1-Boc Piperazine should be stored in a cool, dry place away from light and moisture. Exposure to humidity or strong acids can lead to premature deprotection of the Boc group.
- Safety Precautions: Although not highly toxic, 1-Boc Piperazine may cause skin or eye irritation and should be handled with gloves and protective eyewear. Avoid inhalation of dust or direct contact with skin.
Deprotection of Boc Group
One of the key features of 1-Boc Piperazine is the ability to selectively remove the Boc group under controlled conditions. The deprotection process typically involves:
- Acidic Treatment: Treating the compound with trifluoroacetic acid (TFA) or HCl removes the Boc group, yielding free piperazine.
- Mild Conditions: The deprotection usually occurs under mild conditions, making it compatible with sensitive substrates or complex molecules.
The deprotection of Boc groups can be tuned to occur at specific points in a synthetic route, allowing for the construction of complex molecules with multiple functional groups.
Advantages of Using 1-Boc Piperazine
- Selectivity: The Boc group provides selectivity during chemical reactions, allowing the chemist to control the site of reaction on the piperazine ring.
- Stability: It is stable under most synthetic conditions, especially in basic environments, and can be carried through multiple steps without degrading.
- Wide Utility: It is a versatile reagent that finds applications in pharmaceuticals, materials science, and fine chemical synthesis. Its easy deprotection and robust nature make it a preferred protecting group for many synthetic strategies.
- Simple Deprotection: The Boc group can be removed without causing damage to other sensitive parts of the molecule, enabling multi-step syntheses with minimal side reactions.
Conclusion
1-Boc Piperazine is a highly useful intermediate in organic synthesis, particularly in the pharmaceutical industry, where it plays a pivotal role in the selective protection of nitrogen atoms. Its stability, ease of deprotection, and versatility make it a key reagent for the development of complex molecular architectures, including drugs and peptides. Its importance continues to grow in various fields of chemistry due to its reliable performance and compatibility with a wide range of reactions.
Bottom of Form